$1.3M to improve urea production and reduce carbon dioxide emissions
Rather than contributing to emissions, the production of an essential fertilizer could consume carbon dioxide, and a U-M team will explore such a method.

Rather than contributing to emissions, the production of an essential fertilizer could consume carbon dioxide, and a U-M team will explore such a method.
EXPERTS:
The production of the fertilizer urea is one of the largest carbon dioxide emitters in the chemical industry, but it doesn’t have to be that way. With $1.3 million in funding from the W. M. Keck Foundation, a new, more sustainable approach for producing urea will be tested at the University of Michigan.
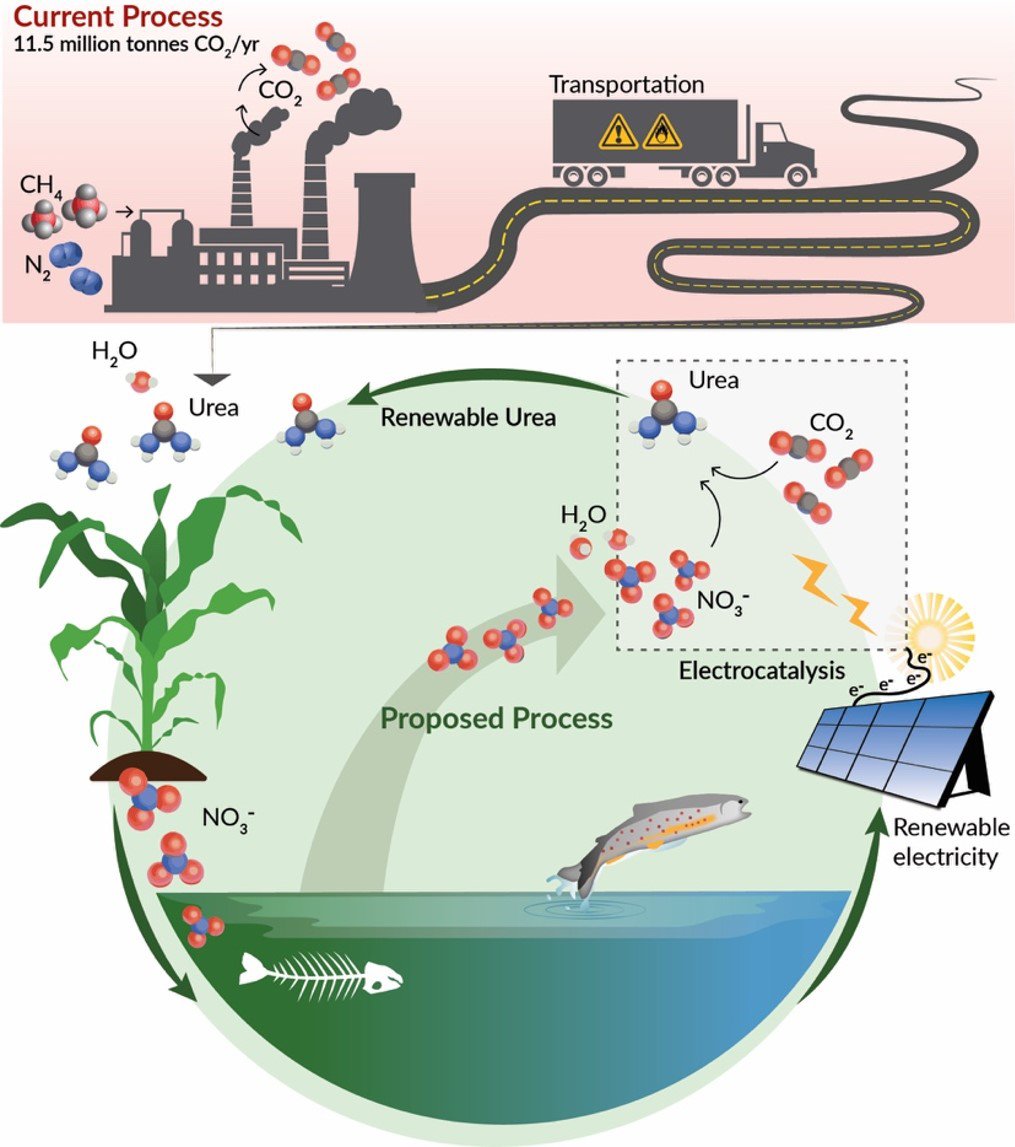
12.5 million tons of carbon dioxide (CO2) are emitted annually by the current process for producing urea (CH4N2O), making it one of the largest CO2 emitters in the chemical industry. The high CO2 emissions stem from producing ammonia (NH3) as a middle step, which requires significant energy for its production.
This project aims to instead assemble the carbon, hydrogen, nitrogen and oxygen atoms in urea by combining water, CO2 and nitrate (NO3-) with electricity. This process, known as co-reduction, directly consumes carbon dioxide as well as nitrate, which is among the most prevalent wastewater pollutants in the world. Nitrate runoff from agriculture and industry leads to out-of-control bacteria and algae growth in aquatic ecosystems, causing dead zones and contaminating drinking water sources. The team proposes to use nitrates from industrial and agricultural waste in their process while capturing carbon dioxide from the air or from sources like smokestacks.
“Directly producing urea from the co-reduction of NO3– and CO2 would remediate two harmful pollutants while producing an essential fertilizer product,” said Bryan Goldsmith, assistant professor of chemical engineering, who leads the project. “This work has the potential to create a paradigm shift in the urea production process and address sustainability.”
The process could also be more energy efficient than current processes, and can be powered with renewable electricity for net-zero emissions. The carbon capture would offset the CO2 emitted from soil when the urea is applied as a fertilizer. However, the carbon dioxide and nitrate will need a new kind of catalyst and a tailored reaction environment to bring them together efficiently, and this is what the team intends to develop.
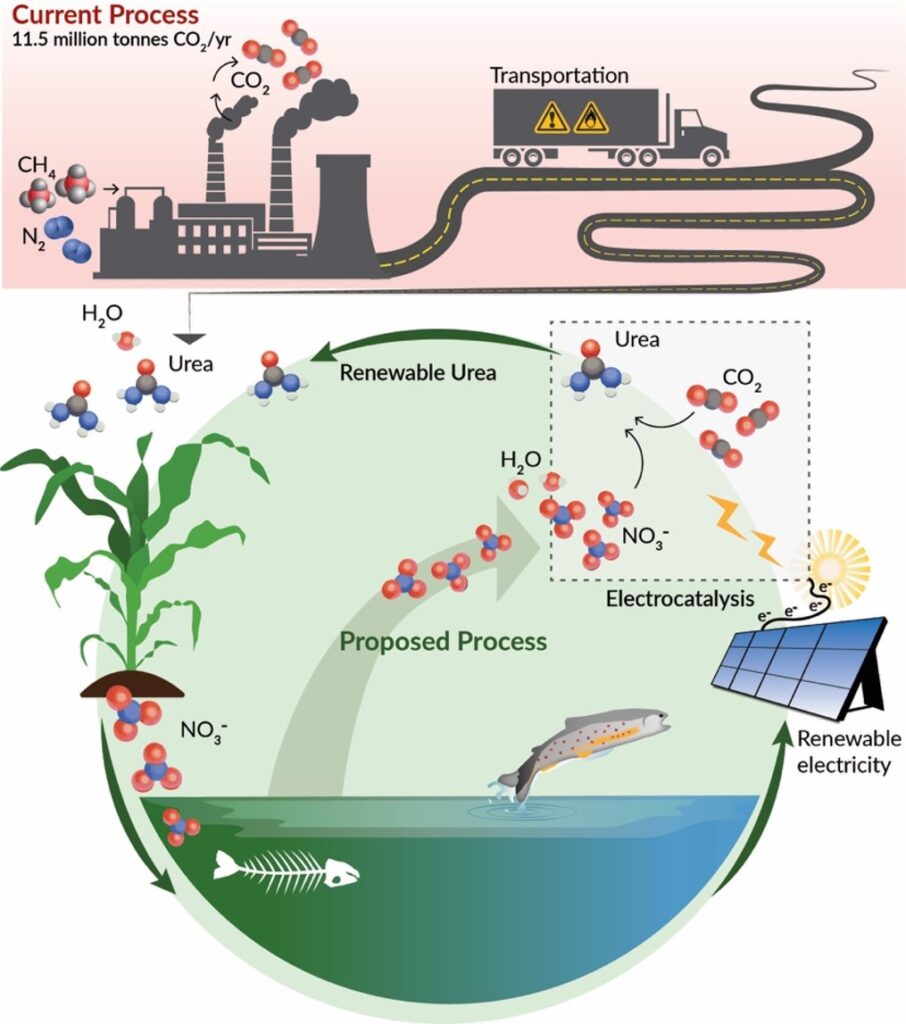
Prior attempts to electrochemically produce urea have focused on coupling CO2 and nitrogen (N2), but researchers found that the stable bond between the nitrogen atoms in N2 is hard to break, making this reaction too energy-intensive and the process unfeasible. Because the bonds between the nitrogen and oxygen atoms in NO3– are less stable, it should take less energy to break them when combining NO3– with CO2.
The Michigan team plans to bring about that reaction with catalyst-polymer composites. The polymers will guide the carbon dioxide, nitrate and just the right amount of hydrogen from water to the reaction sites on the catalysts. The catalysts will be alloys of pairs of metals, in various compositions, that will be able to react with both CO2 and NO3-.
Electricity will help remove oxygen from the CO2 and NO3– so that they can be combined at the catalyst reaction sites. By controlling the delivery of the reactants and electricity, the team will have a unique level of control over the reaction.
This approach could also transform the production of compounds such as amides and amines used for chemical synthesis, pharmaceuticals and agriculture—ultimately leading to cleaner and more economical processes that increase the sustainability of food systems and improve the planet’s health.
“The support from Keck allows us to work on the many different aspects of this reaction in a concerted way, rather than each working on the different components separately. This affords us a unique opportunity that we are very excited about,” said Nirala Singh, an assistant professor of chemical engineering and co-investigator of the study.

Other co-investigators on the team are Suljo Linic, the Martin Lewis Perl Collegiate Professor of Chemical Engineering and a professor of integrative systems and design; Jovan Kamcev, an assistant professor of chemical engineering and macromolecular science and engineering; Rohini Bala Chandran, an assistant professor of mechanical engineering; and Charles McCrory, an associate professor of chemistry and macromolecular science and engineering. In previous work, the team has employed their complementary expertise to conduct the foundational research for this new application.
Members of this research team have received numerous prestigious awards in the fields of chemistry, engineering, and catalysis including recognitions from the National Science Foundation, U.S. Department of Energy, the American Chemical Society, and the American Society of Mechanical Engineers.
The W. M. Keck Foundation was established in 1954 in Los Angeles by William Myron Keck, founder of The Superior Oil Company. One of the nation’s largest philanthropic organizations, the W. M. Keck Foundation supports outstanding science, engineering and medical research. The Foundation also supports undergraduate education and maintains a program within Southern California to support arts and culture, education, health and community service projects.